Spending enough time in the sun without adequate protection can leave us looking and presumably feeling like a lobster ready for the plate.
The conventional explanation for the skin's painful inflammation response involves a cascade of effects triggered by breaks in the tissue's DNA.
It now seems we might have had that key detail incorrect all along. According to a new study involving mice and human skin cells, the first moments of sunburn are a little different from what anybody expected.
"Sunburn damages the DNA, leading to cell death and inflammation. So the textbooks say," says Anna Constance Vind, a molecular biologist from the University of Copenhagen who led an investigation that challenges what we thought we knew about sun damage.
"But in this study we were surprised to learn that this is a result of damage to the RNA, not the DNA that causes the acute effects of sunburn."
The term sunburn is something of a misnomer. Unlike low-level thermal burns, which result from heat making a mess of your body's proteins, sunburn damage is caused by prolonged doses of shorter-wavelength 'B' type ultraviolet radiation.
Regardless of how the damage is caused, the result is the same: a variety of cellular stresses alert the immune system to a threat, setting off a domino effect of chemical sirens that widen some blood vessels, restrict others, and elevate sensitivity to pain.
Pinning down precisely what those triggering factors are can be messy. Heat itself can induce responses, for example. Sudden shifts in water, reactive species of oxygen released by broken compounds, and simple fractures in the cells themselves can all send signals to the immune system that it needs to act fast.
As the bonds between nucleic bases can absorb photons of ultraviolet B to the point of breaking and reconfiguring, it's long been presumed that DNA damage, in association with other forms of cellular damage, is critical in those first moments of signaling.
"DNA damage is serious as the mutations will get passed down to progenies of the cells, RNA damage happens all the time and does not cause permanent mutations," says Vind.
"Therefore, we used to believe that the RNA is less important, as long as the DNA is intact. But in fact, damages to the RNA are the first to trigger a response to UV radiation."
Vind and her colleagues demonstrated this by using mice genetically engineered to be missing a stress response protein called ZAK-alpha, which by binding with cellular machinery that translates strings of messenger RNA into proteins can ring the alarm bells when the translation process isn't going to plan.
Dosing mice with and without ZAK-alpha with ultraviolet B or an antibiotic that also triggers the protein into responding reveals the RNA-mediated stress response is critical in creating sunburn's symptoms.
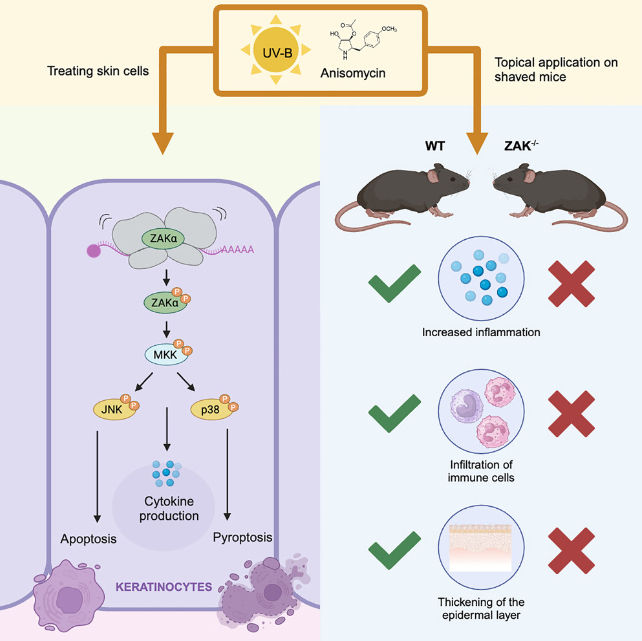
Together with a series of laboratory experiments on human skin cells designed to test the consequences of UV-induced DNA damage, the team showed significant changes to a cell's messenger RNA caused the cell to shut down and the immune system to respond.
Without ZAK-alpha, mice exposed to ultraviolet B radiation didn't get burned as normal, suggesting RNA damage may also be key to our bodies' sensitivity to the sun.
While damage to the central DNA library may be a greater cause for concern, monitoring the cell's messenger system might give cells the edge in a more rapid response to the threat of radiation.
"The fact that the DNA does not control the skin's initial response to UV radiation, but that something else does and that it does so more effectively and more quickly, is quite the paradigm shift," says Vind.
By continuing to explore the consequences of RNA damage and its role in stress responses, we might find ways to better treat sunburn and other conditions exacerbated by sunlight.
This research was published in Molecular Cell.