The key to stopping Alzheimer's may lie with a woman who never had the disease.
Despite having a strong genetic risk, a woman who carried two copies of a rare genetic variant linked with late onset Alzheimer's called APOE3 Christchurch appeared resistant to the disease's cognitive decline.
Now scientists have observed how mice with a similar set of genetic mutations responded to Alzheimer's-like conditions.
Similar to the human case, the mice appeared to have fewer neurological defects associated with advanced stages of the disease, with the key factor being how the brain's cleaning cells (microglia) respond to the disease's pathology.
This provides fresh hope for the development of treatments for Alzheimer's that focus on eliciting these particular responses.
The research team from the Washington University School of Medicine says this response helps break the connection between the early, symptom-free stage of Alzheimer's, and the late stage of cognitive decline.
Both genetic – called autosomal dominant Alzheimer's disease (ADAD) – and nongenetic forms of Alzheimer's take about 30 years to develop. There are no symptoms for the first 20 years or so as amyloid builds up in the brain slowly.
When amyloid levels in the brain reach a critical point, several destructive processes begin to work together. A protein called tau starts to tangle up and spread, slowing down the brain's metabolism and causing the tissues to shrink, leading to cognitive decline.
"One of the biggest unanswered questions in the Alzheimer's field is why amyloid accumulation leads to tau pathology," neurologist David Holtzman says.
"Any protective factor is very interesting, because it gives us new clues to how the disease works."
An extended family in Colombia had been dealing with ADAD for many generations, with symptoms beginning in half of the family members in their 40s. In this family's case, the disease was triggered by a mutation in a gene called presenilin-1, associated with an increased tendency to form amyloid plaques, with amyloid buildup starting around age 20.
One person in that family achieved what seemed impossible: she stayed cognitively healthy into her 70s even though she had inherited the presenilin-1 mutation.
The woman appeared to be the only one in the group with two copies of APOE3 Christchurch (APOE3ch) which was proposed as an explanation for her good luck. Those carrying only a single copy of APOE3ch still exhibited signs of cognitive deterioration at a younger age.
A study in 2019 speculated that the woman's extra mutations were delaying the process by slowing tau's rapid spread.
"This woman was very, very unusual in that she had amyloid pathology but not much tau pathology and only very mild cognitive symptoms that came on late," Holtzman explains. "This suggested to us that she might hold clues to this link between amyloid and tau."
But since this specific set of genetic mutations has only been recorded in one person in the world, it's been impossible to determine whether other factors might be involved in her remarkable cognitive health.
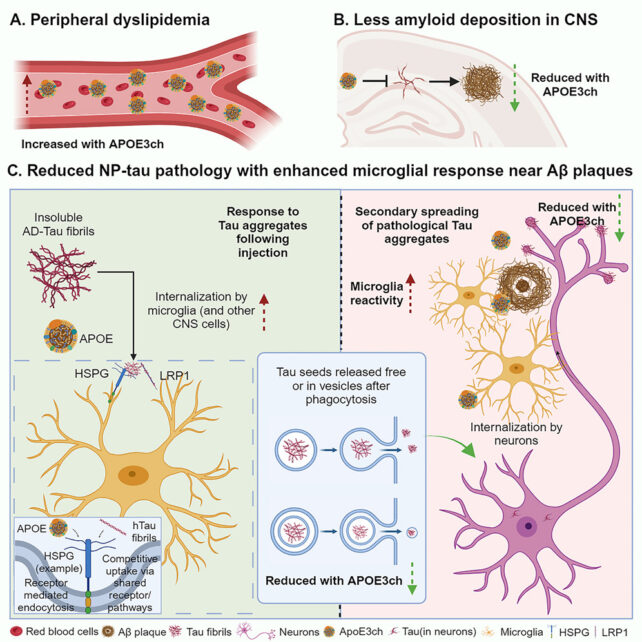
So Holtzman and colleagues studied mice that were genetically modified to produce excess amyloid, and introduced a gene with the APOE3ch mutation. They then injected a small amount of tau – expected to cause problems in brains already filled with amyloid.
In the mouse models, like in the case of the Columbian woman, the tau didn't spread as expected. The reason: microglia around the amyloid plaques were super active and efficient in cleaning up the protein.
"These microglia are taking up the tau and degrading it before tau pathology can spread effectively to the next cell," says Holtzman.
"That blocked much of the downstream process; without tau pathology, you don't get neurodegeneration, atrophy and cognitive problems."
The protective effects of APOE3ch aren't clear in late onset Alzheimer's though, and could vary depending on a person's ancestry or if other genetic mutations are involved. This is an important area to be studied further, the team adds.
"If we can find a way to mimic the effects of the APOE Christchurch mutation," Holtzman says, "we may be able to stop people who already are on the path to Alzheimer's dementia from continuing down that path."
The study has been published in Cell.