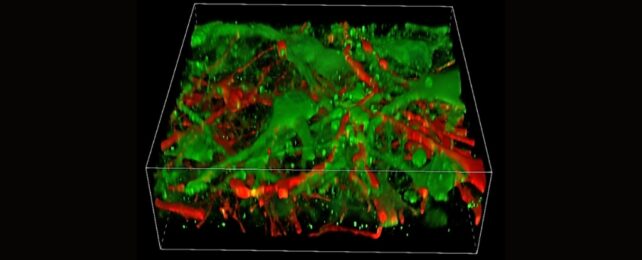
Researchers have created the first functional 3D-printed brain tissue that can develop and form connections in the same way as real human brain tissue.
This remarkable accomplishment by a team at the University of Wisconsin–Madison provides neuroscientists with a new tool for studying communication between brain cells and other parts of the human brain, potentially leading to better ways of treating diseases like Alzheimer's and Parkinson's.
"It could change the way we look at stem cell biology, neuroscience, and the pathogenesis of many neurological and psychiatric disorders," says neuroscientist Su-Chun Zhang, senior author of a new paper describing the research.
Zhang and his team say many labs should be able to use their new method as it doesn't need special bio-printing equipment. In addition, the tissue is easy to keep healthy and can be studied with microscopes and other equipment typically found in most laboratories.
3D bioprinting – a computer-guided process that builds layers of materials, cells, and other components to build living structures – has huge potential for creating tissues that replicate, and in some cases even replace the real deal.
"Because we can print the tissue by design, we can have a defined system to look at how our human brain network operates," says Zhang. "We can look very specifically at how the nerve cells talk to each other under certain conditions."
To understand the human brain networks for studying health and diseases, we need a reliable model of living human neural tissues, the researchers explain, since animal models can't fully replicate the brain's complexity.
But it's challenging to print functional human brain tissue and so far most 3D-printed tissues lack proper connections between cells. Neurons need to be able to mature while keeping the tissue structure intact, and supporting cells like astrocytes are essential for the tissue to function properly.
Earlier attempts used non-biodegradable scaffolds that preventing neural cells from easily migrating. Instead of the usual vertical layering, the team used horizontal layering of neurons derived from induced pluripotent stem cells, placed in a softer 'bio-ink' gel than in previous methods.
Their printed tissue cells can form brain-like networks within and between layers in just a few weeks. Neurons communicate, send signals, use neurotransmitters, and even form networks with added supporting cells.
"The tissue still has enough structure to hold together but it is soft enough to allow the neurons to grow into each other and start talking to each other," explains Zhang.
"Even when we printed different cells belonging to different parts of the brain, they were still able to talk to each other in a very special and specific way."
He says studying one thing at a time means missing crucial components because the brain functions in networks. Printing brain tissue this way allows for a clearer observation of cell interactions.
"We printed the cerebral cortex and the striatum and what we found was quite striking," Zhang says.
They found that the axons projecting in the printed brain tissue mirror the human brain pattern, where cortical neurons project axons to the striatum.
The precision of this 3D-printing method allows control over cell types and arrangements, unlike miniature lab-grown organs used for brain research called brain organoids.
The prototype can't control the direction of mature neurons though, and the printed tissue lacks the natural structure seen in brain organoids. But Zhang and colleagues say it complements organoids as a useful way to study the brain under different conditions.
"It can be used to look at the molecular mechanisms underlying brain development, human development, developmental disabilities, neurodegenerative disorders, and more," Zhang explains.
The team hopes to improve their process to create more specific brain tissues with guideable cells.
The study has been published in Cell Stem Cell.