Very few painkillers are safe to use during pregnancy, and yet one of the only available options has become embroiled in an international debate in recent years.
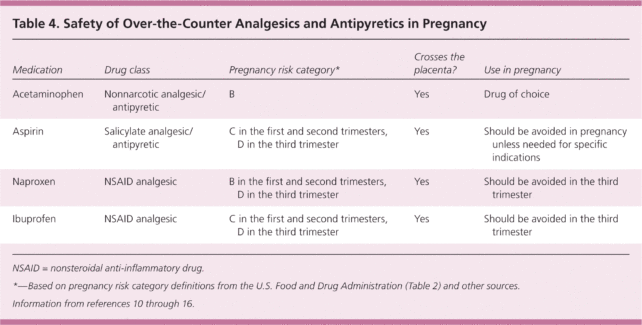
Acetaminophen, also known as paracetamol, is generally considered to be the safest painkiller to use during pregnancy, and yet emerging research that has linked the drug to attention deficit hyperactivity disorder ( ADHD) suggests there may be overlooked risks to early brain development.
In a small new study, researchers in the US tracked bloodstream levels of acetaminophen in 307 Black women during their pregnancy. They found those who used acetaminophen later gave birth to children more than three times as likely to receive an ADHD diagnosis.
For daughters, exposure to acetaminophen in the womb was linked to a more than six-fold increase in the risk of ADHD within the first ten years of life.
While that sounds concerning on the surface, these initial results are not conclusive and should not scare off the large percentage of people who rely on acetaminophen during pregnancy for pain or fever. Especially since robust evidence shows both of those symptoms can be threats to a developing fetus if left untreated.
As with any medicine, the benefits of acetaminophen must be balanced by the risks. Unlike its pros, however, the long-term cons are not as well researched.
"This medication was… approved decades ago, and may need reevaluation by the FDA," argues pediatrician Sheela Sathyanarayana from UW Medicine.
"Acetaminophen was never evaluated for fetal exposures in relation to long-term neurodevelopmental impacts."
In recent years, several other epidemiology studies have found associations between acetaminophen use during pregnancy and ADHD outcomes in children, and yet some studies have produced conflicting results. All of these investigations are purely correlational and their findings are not a cause for alarm, some scientists argue, but rather, alertness.
Because of the current study's small sample size, the data are probably not robust enough to change the minds of officials at the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), the American College of Obstetricians and Gynecologists (ACOG), the Society of Obstetricians and Gynecologists of Canada, and the Society for Maternal-Fetal Medicine – all of whom maintain that acetaminophen poses minimal risk when using the lowest dose as needed during pregnancy.
For instance, the current study does not account for factors "like the mother's reason for taking [paracetamol], such as headaches or fevers or pains or infections, which we know are risk factors for adverse child development," statistical geneticist Viktor Ahlqvist from Sweden's Karolinska Institute, who was not involved in the current research, told Grace Wade at New Scientist.
Nevertheless, lead author Brennan Baker, from Seattle Children's Research Institute, thinks it may be time for the FDA to take another look at acetaminophen's safety during pregnancy.
The last time the FDA did so was in 2015, when officials declared there was inconclusive evidence to connect acetaminophen use in pregnancy and ADHD in children.
"Most of the prior studies asked women to self-report whether they had taken Tylenol or anything that contained acetaminophen," explains Baker.
In 2020, two studies (one led by Baker) measured acetaminophen levels in newborns and found that higher levels of the painkiller were linked with ADHD in childhood. One of these also found a link to autism spectrum disorder ( ASD).
In light of this initial data, an international team of 91 scientists, clinicians, and public health professionals came together to urge 'precautionary action'.
"[Acetaminophen] is an important medication and alternatives for treatment of high fever and severe pain are limited," they wrote in a Consensus Statement for Nature Reviews Endocrinology in 2021.
"We recommend that pregnant women should be cautioned at the beginning of pregnancy to: forego acetaminophen unless its use is medically indicated; consult with a physician or pharmacist if they are uncertain whether use is indicated and before using on a long-term basis; and minimize exposure by using the lowest effective dose for the shortest possible time."
In response to the 2021 commentary, officials at ACOG held firm.
"Neurodevelopmental disorders, in particular, are multifactorial and very difficult to associate with a singular cause. The brain does not stop developing until at least 15 months of age, which leaves room for children to be exposed to a number of factors that could potentially lead to these issues," they wrote in 2021.
"ACOG's clinical guidance remains the same and physicians should not change clinical practice until definitive prospective research is done."
A study on 307 pregnant people that needs to be replicated again is probably not going to cut it.
"The conflicting results means that more research is needed," says Baker.
The study was published in Nature Mental Health.
