Scientists have invented a new method for turning carbon dioxide into a liquid fuel that can efficiently store energy in fuel cells.
The fuel could one day be the future of green transport, cramming more energy into the tank than the same volume of hydrogen while also serving as a building block for a whole chemical production industry.
In recent years, a new kind of technology based on formic acid has attracted attention as the next generation of fuel cells.
Formic acid isn't typically what comes to mind when we think of the fuel of the future. Found naturally contributing to the pain of bee and ant stings, it is a formidable energy carrier. It just currently takes a lot of effort to concentrate into a useful form.
Engineers at Rice University in Houston, Texas, have rethought the entire production process and come up with a clever method to do away with some of the more involved steps, making the process far more efficient.
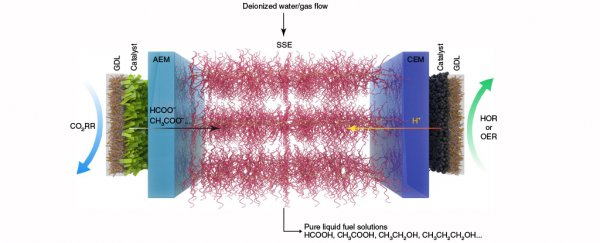
"Usually people reduce carbon dioxide in a traditional liquid electrolyte like salty water," says chemist Haotian Wang.
Those dissolved salts help convert the gas into a molecule that stores energy. But once you've got your fuel, you also have a thick briny soup to deal with, and sifting out the formic acid is painstaking work.
"So we employed solid electrolytes that conduct protons and can be made of insoluble polymers or inorganic compounds, eliminating the need for salts," says Wang.
Replacing the electrolyte with a solid matrix was just one improvement. The second was coming up with a robust catalyst to speed up the conversion process. A common challenge is keeping a catalyst right where you want it, without it degrading and needing to be replaced over time.
Bismuth is just the catalyst for the job. Bulkier than other metals capable of the same task, it won't move about as easily. You just need enough material to turn a lab-test into an industry.
The research team found a solution here as well.
"Currently, people produce catalysts on the milligram or gram scales," says the investigation's lead author, Chuan Xia.
"We developed a way to produce them at the kilogram scale."
The resulting device is engineered to channel the carbon dioxide through the catalyst where it transforms into a negatively charged molecule called formate.
From there it diffuses into the solid electrolyte core, where it meets hydrogen ions released from a second catalytic reaction with water, resulting in a highly concentrated solution of formic acid.
So far, the process has been shown to convert about 42 percent of the electricity from a power source into a chemical form that can be used in fuel cells.
This electricity can easily come from a renewable source, such as a photovoltaic cell or a wind turbine, providing a neat new way to store energy from otherwise variable power supplies.
"It's also fundamental in the chemical engineering industry as a feedstock for other chemicals, and a storage material for hydrogen that can hold nearly 1,000 times the energy of the same volume of hydrogen gas, which is difficult to compress," says Wang.
"That's currently a big challenge for hydrogen fuel-cell cars."
Mining the atmosphere for carbon dioxide in order to satisfy our growing energy demands amid climate change sounds like a winning solution.
Technology is leaping ahead in finding ways to use our overabundance in greenhouse gases to wean ourselves off polluting fuels, from finding ways to use it to charge batteries to taking a leaf from nature's page and improving on photosynthesis itself.
Meanwhile, other researchers are keen to turn it into a solid material resource. If not simply bury the stuff deep underground in rock form again.
However we do it, it's going to need to satisfy the economy before it does our sense of self-preservation.
This research was published in Nature Energy.