In the eternal arms race between bacteria and antibiotics, deadly superbugs with resistance to humanity's most vital life-saving medicines continue to emerge and evolve.
It's a growing crisis, but thankfully we are not entirely powerless against the scourge of antibiotic resistance.
In medical scenarios where frontline treatments fail to help patients, doctors can turn to so-called drugs of last resort – treatments set aside until the eleventh hour has come, after prioritized therapies haven't worked out.
Drugs of last resort may be held back for a number of reasons, including side effects, cost factors, patient considerations, and more.
In the antibiotics context, there's an additional pretext: We don't want highly resistant bacteria to learn how to resist these drugs too, so clinicians limit their use wherever possible.
Colistin is one such medicine. One of only 17 'Reserve Group' antibiotics on the World Health Organization's List of Essential Medicines, colistin was first discovered in the 1940s, and is used as a last resort against multi-drug-resistant pathogens such as Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
Colistin is not perfect, however. The drug's toxicity can produce a number of serious side effects, putting a constraint on dosage levels – and at low doses it's not always effective in patients.
Because of this, there's a pressing need to know more about how colistin functions. Not only to see if there are ways we can boost its efficacy, but also maybe to save the drug itself: The first signs of bacterial resistance to colistin began to emerge a decade ago, and have now spread around the world.
"As the global crisis of antibiotic resistance continues to accelerate, colistin is becoming more and more important as the very last option to save the lives of patients infected with superbugs," says microbiologist Akshay Sabnis from Imperial College London in the UK.
"By revealing how this old antibiotic works, we could come up with new ways to make it kill bacteria even more effectively, boosting our arsenal of weapons against the world's superbugs."
Strangely enough, for a drug that's been around for over 70 years, the mechanisms by which colistin ultimately kills bacteria have remained somewhat mysterious. Until now.

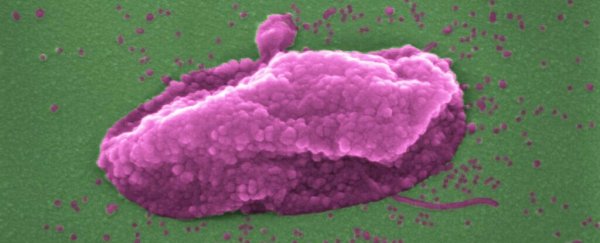
Above: The superbug Pseudomonas aeruginosa, before and after being 'popped' by colistin. (Imperial College London)
In a new study led by Sabnis, researchers conducted experiments with multiple strains of bacteria to investigate how colistin functions at the molecular level.
Colistin is an example of a polymyxin antibiotic, which works by binding to the outer cellular membrane of gram-negative bacteria, ultimately disrupting the outer membrane and then the inner membrane (aka the cytoplasmic membrane).
In so doing, this kills the microbes – punching holes in their bodies to effectively make them pop like balloons.
In this process, colistin targets molecules called lipopolysaccharides in the bacterial outer membrane, but exactly how the antibiotic disrupted the inner barrier was less certain, since the inner membrane contains much lower levels of lipopolysaccharides.
Thanks to new tests with E. coli strains, the researchers confirmed that lipopolysaccharide disruption is also what's responsible for destroying the inner cell membrane, even though the molecule's presence there is much lower.
"It sounds obvious that colistin would damage both membranes in the same way, but it was always assumed colistin damaged the two membranes in different ways," says molecular microbiologist Andy Edwards, the senior author of the study.
"By changing the amount of lipopolysaccharides in the inner membrane in the laboratory, and also by chemically modifying it, we were able to show that colistin really does puncture both bacterial skins in the same way – and that this kills the superbug."
Even more promisingly, the researchers discovered a way to augment colistin's ability to disrupt the inner membrane, thanks to an experimental antibiotic called murepavadin, which boosts the levels of lipopolysaccharides in bacterial inner membranes.
Hypothetically speaking, pairing colistin with murepavadin could give the former more molecular bullseyes on the balloon to target, and subsequent experiments seemed to bear this line of reasoning out.
In experiments with mice infected by P. aeruginosa bacteria, treatment with either colistin or murepavadin by itself had very little immediate effect on bacterial load in the animals, but the combination therapy produced a roughly 500-fold reduction in bacterial colony-forming units in just three hours.
While murepavadin is an experimental drug not yet cleared for clinical use in human patients, the new findings indicate a potent path forward – where an old antibiotic and a new ally join forces against a common enemy.
"It is anticipated that a combination of colistin and murepavadin could enhance the low treatment efficacy of polymyxin antibiotics and may also limit the toxic side effects associated with both compounds by enabling the use of lower doses of the drugs," the authors write in their study.
"Modulation of lipopolysaccharide levels in the cytoplasmic membrane can enhance colistin activity, providing the foundations for new approaches to enhance the efficacy of this antibiotic of last resort."
The findings are reported in eLife.